
Reflow Transfer for Conformal Three-Dimensional Microprinting
December 23, 2022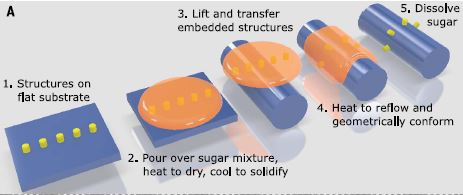

The overall technique is explained in the top picture, while the result can for example be the word "NIST" written on a human hair, shown to the right.
Check out what else can be done with this technique in the just published article "Zabow G, Science 378, 894–898 (2022)".
Special JMMM Issue Available after the 2022 Meeting of Scientific and Clinical Applications of Magnetic Carriers
December 03, 2022 The special JMMM issue after the 2022 meeting is now available, check it out here:
The special JMMM issue after the 2022 meeting is now available, check it out here:
https://www.sciencedirect.com/journal/journal-of-magnetism-and-magnetic-materials/special-issue/10CRH1HLML1
Seventeen original articles are now available at this link. Get a coffee and some cake, and look through these interesting articles, they are well worth it!
The editors for this special issue were Silvio Dutz, Lucia Gutierrez and Maciej Zborowski. Thank you very much for doing this!
Surgical Procedures to Lengthen Your Legs Use Magnetic Force for Extension
November 02, 2022As we find all magnetic procedures interesting, here is a new method for the leg lengthening of people. A 25 to 30 cm long rod between half and one centimeter is implanted surgically into the center of the long leg bones. Every day after that, the rod which contains some internal gears is lengthened by about 1 mm per day and thus stretches the leg by the same distance. The mechanical movement by these gears is induced by an external magnetic field. The body seems to fill in the stretched distance by physiological bone and other tissues. Have a look at some of the details, unfortunately described not in the most scientific way:
https://www.businessinsider.com/limb-leg-lengthening-surgeon-la-how-it-works-patients-2022-11
Sirine El Mousli and Melody Perret Won A Photo Competition
June 28, 2022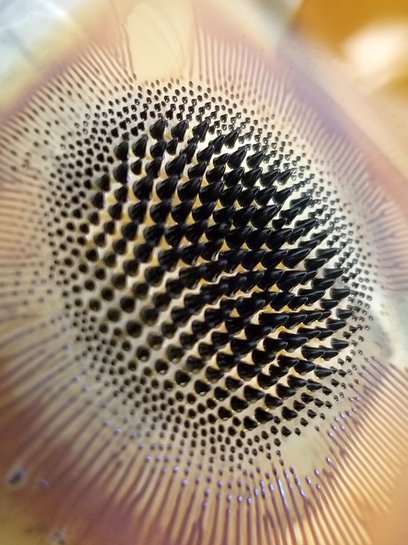 Sirine El Mousli and Mélody Perret from the PHENIX lab at the Sorbonne University won a the LaboPhoto competition of the French Chemistry Society with their beautiful ferrofluid picture. The title of the picture is "Fighting Cancer With Ferrofluids". Congratulations to both authors for this achievement!
Sirine El Mousli and Mélody Perret from the PHENIX lab at the Sorbonne University won a the LaboPhoto competition of the French Chemistry Society with their beautiful ferrofluid picture. The title of the picture is "Fighting Cancer With Ferrofluids". Congratulations to both authors for this achievement!
Magnetic Carrier Meeting 2022 in London, England
June 14, 2022.JPG)
Magnetic Carrier Meeting 2022
from June 14-17, 2022
After skipping the 2020 meeting due to COVID-19, the 13th International Conference on the Scientific and Clinical Applications of Magnetic Carriers in London at the University College London (UCL) from June 14-17, 2022 was a great success. Exactly 209 experts and novices in the world of magnetic particles and their applications took part in this 4-day conference. The organization with the help of Prof. Nguyen Thanh's local team was flawless, the food was good, and reception and poster sessions were very animated. Thank you all for being with us at this wonderful meeting!
For more details about the 2022 meeting, go to the main meeting website.
Magnetic Cube Microbots
May 12, 2022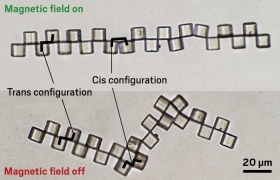 Janus cubes, polymer microparticles coated with metal on one side, self-assemble into various structures under the influence of a magnetic field, and manipulating the magnetic field turns the structures into microbots for use in drug delivery, in cell measurement, or as miniature actuators. Orlin Velev, a chemical engineer at North Carolina State University, outlined his group’s work. The researchers use photolithography to create polymer cubes about 10 µm across, then coat one side with a 10-nm layer of chromium topped by a 100-nm layer of cobalt. Placing the cubes between two electromagnets causes them to align and form a chain that stays connected after the field is turned off. Oriented one way (trans), the north-south poles of adjoining magnets align into a stiff connection. In the other orientation (cis), they can flip back and forth, so the chains fold and unfold when a magnetic field is applied. The group has made the chain fold around a cell and applied a magnetic gradient to move the captured cell. Another group at Swiss Federal Institute of Technology (ETH), Zurich, has shown it can control the microbots inside a rabbit eye as a possible microsurgical tool. Velev is studying if by squeezing a cell to measure its stiffness, he can determine whether it is healthy or infected with a virus. A related application involves contracting and expanding the chains to act as microactuators and tiny muscles.
Janus cubes, polymer microparticles coated with metal on one side, self-assemble into various structures under the influence of a magnetic field, and manipulating the magnetic field turns the structures into microbots for use in drug delivery, in cell measurement, or as miniature actuators. Orlin Velev, a chemical engineer at North Carolina State University, outlined his group’s work. The researchers use photolithography to create polymer cubes about 10 µm across, then coat one side with a 10-nm layer of chromium topped by a 100-nm layer of cobalt. Placing the cubes between two electromagnets causes them to align and form a chain that stays connected after the field is turned off. Oriented one way (trans), the north-south poles of adjoining magnets align into a stiff connection. In the other orientation (cis), they can flip back and forth, so the chains fold and unfold when a magnetic field is applied. The group has made the chain fold around a cell and applied a magnetic gradient to move the captured cell. Another group at Swiss Federal Institute of Technology (ETH), Zurich, has shown it can control the microbots inside a rabbit eye as a possible microsurgical tool. Velev is studying if by squeezing a cell to measure its stiffness, he can determine whether it is healthy or infected with a virus. A related application involves contracting and expanding the chains to act as microactuators and tiny muscles.
New MPI Review
April 24, 2022.JPG)
Check it out, the article is freely available at https://pubs.rsc.org/en/content/articlepdf/2022/nr/d1nr05670k.
New Review About Ferrofluids and Bio-Ferrofluids: Looking Back and Stepping Forward
March 21, 2022
Check it out here:
Socoliuc V, Avdeev MV, Kuncser V, Turcu R, Tombácz E, Vékás L (2022). Ferrofluids and bio-ferrofluids: looking back and stepping forward. Nanoscale, in print.
For more information, check out our Archives.
September 2017
Search this site with the power of
